Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitr
![Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:598/0*gC7TqoG4Szy6QwnB.jpg)
Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![SOLVED: Two complex ions containing Ni are [Ni(NH3)6]2+, which is blue, and [Ni(en)3]2+, which is purple. Which one of these statements is true? The crystal field splitting energy (D) is greater for [ SOLVED: Two complex ions containing Ni are [Ni(NH3)6]2+, which is blue, and [Ni(en)3]2+, which is purple. Which one of these statements is true? The crystal field splitting energy (D) is greater for [](https://cdn.numerade.com/ask_previews/0233c9d6-8ba9-4d87-932d-970ef67d8fc4_large.jpg)
SOLVED: Two complex ions containing Ni are [Ni(NH3)6]2+, which is blue, and [Ni(en)3]2+, which is purple. Which one of these statements is true? The crystal field splitting energy (D) is greater for [
![Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory. Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.](https://haygot.s3.amazonaws.com/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.
1) what is the Oxidation no. Of Co,Fe,Ni in [Co(NH3)6]3+,[Fe(CN)6]4 and [Ni(Co)4]?How? (2) Coordination no. Of [Fe(C2O4)3]3 and [Co(en)3]3+?How?
![SOLVED: [NiOHz)]2+ is green; while [Ni(NH3)]2+ is purple: Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6l2+ absorbs violet light [Ni(OHz)a]2+ absorbs red light. SOLVED: [NiOHz)]2+ is green; while [Ni(NH3)]2+ is purple: Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6l2+ absorbs violet light [Ni(OHz)a]2+ absorbs red light.](https://cdn.numerade.com/ask_images/6feedc9774204ebeabd886be4a6d6f35.jpg)
SOLVED: [NiOHz)]2+ is green; while [Ni(NH3)]2+ is purple: Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6l2+ absorbs violet light [Ni(OHz)a]2+ absorbs red light.
![Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex. Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex.](https://haygot.s3.amazonaws.com/questions/1048239_879440_ans_9590dd8f7eff475e873fdfa26f547f7a.PNG)
Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex.
![Number of complex which are sp^3d^2 hybridised. (i) [Fe)NH3)6] Cl2 (ii) [Mn (NH3)6] Cl2 (ii) [Pt(NH3)6]Cl2 (iv) [Ni(NH3)6]Cl2 Number of complex which are sp^3d^2 hybridised. (i) [Fe)NH3)6] Cl2 (ii) [Mn (NH3)6] Cl2 (ii) [Pt(NH3)6]Cl2 (iv) [Ni(NH3)6]Cl2](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/219048249_web.png)
Number of complex which are sp^3d^2 hybridised. (i) [Fe)NH3)6] Cl2 (ii) [Mn (NH3)6] Cl2 (ii) [Pt(NH3)6]Cl2 (iv) [Ni(NH3)6]Cl2
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1280/0*8wwL8Ru43LyLIIL8.png)
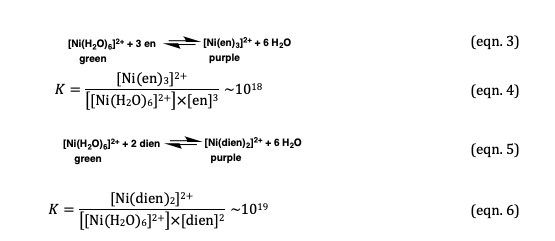
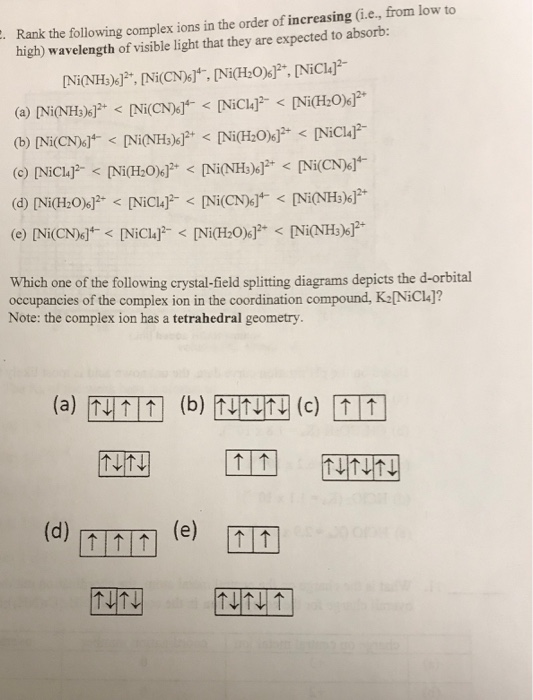

![Solved Or blue color like [Ni(NH3) 6]2+ (aq)? An aqueous | Chegg.com Solved Or blue color like [Ni(NH3) 6]2+ (aq)? An aqueous | Chegg.com](https://media.cheggcdn.com/study/437/437f5cd4-67bf-4a61-bbd0-59efdb460b96/image.png)




![Cr(NH_3)_6]^(3+)` is paramagnetic while `[Ni(CN)_4]^(2-)` is diamagnetic. Explain why?... - YouTube Cr(NH_3)_6]^(3+)` is paramagnetic while `[Ni(CN)_4]^(2-)` is diamagnetic. Explain why?... - YouTube](https://i.ytimg.com/vi/2ukVHaMEd1I/maxresdefault.jpg)
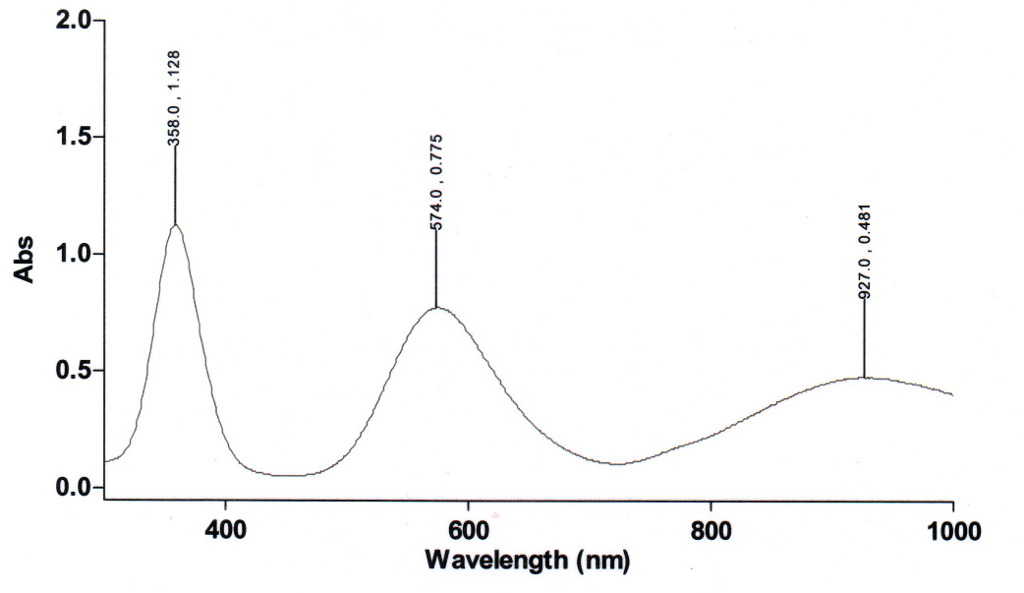
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)
2 PDF) Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2](https://i1.rgstatic.net/publication/257615871_Thermal_decomposition_of_polycrystalline_NiNH36NO32/links/004635278ad7fcd6c2000000/largepreview.png)
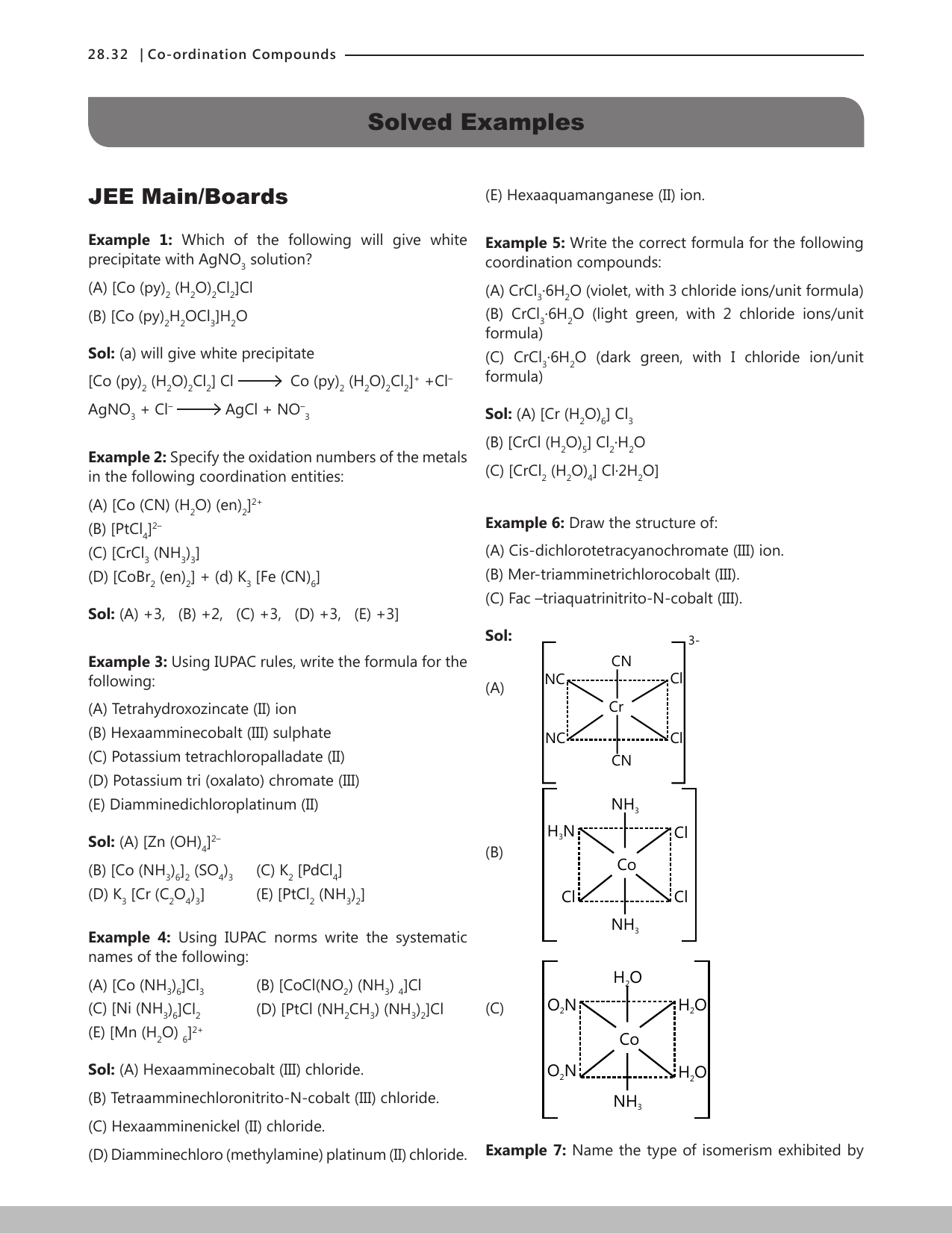
![Solved 6. [Ni(en)]2+ + 6NH3 = [Ni(NH3).]2+ + 3en Show | Chegg.com Solved 6. [Ni(en)]2+ + 6NH3 = [Ni(NH3).]2+ + 3en Show | Chegg.com](https://media.cheggcdn.com/media/e83/e83b4bd2-112b-4266-b86c-7bc56d6d27e3/phpnyZUYa.png)

![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-61bcbbeed69e0a294c52675e216d428f.webp)