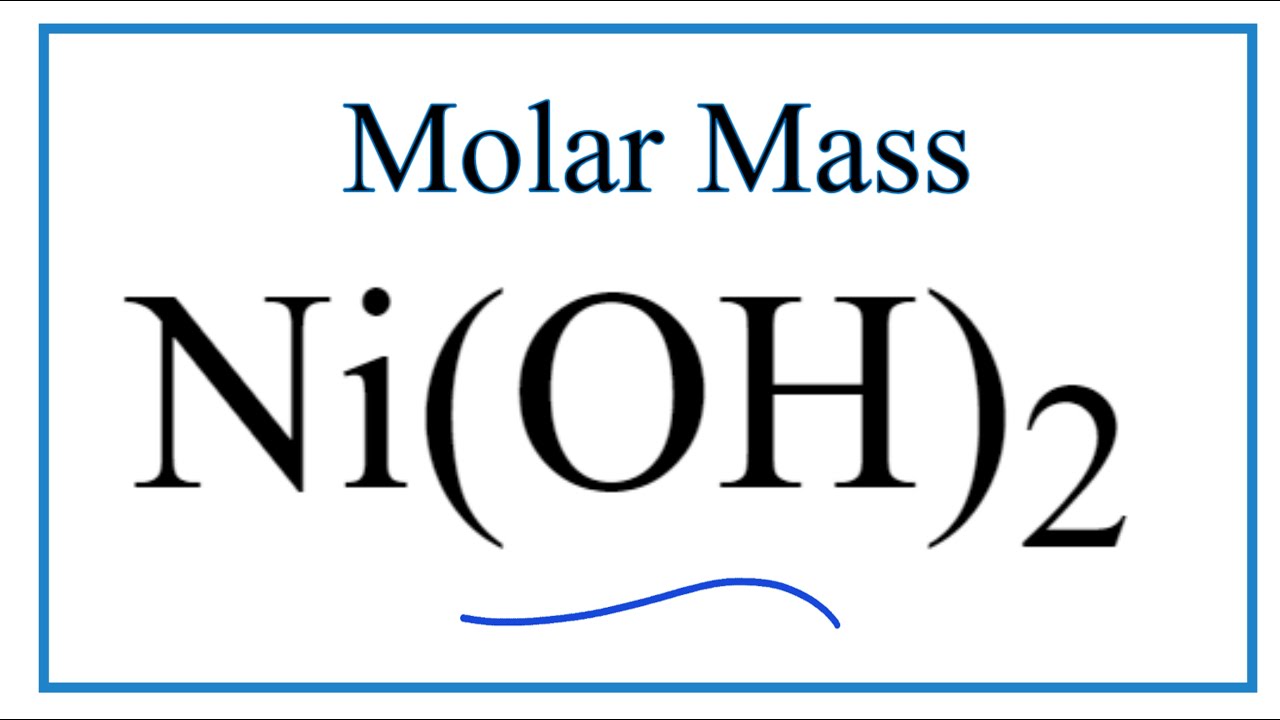
Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni (OH)2-Pt Interfaces | Science

Chemistry lovers - #Metal hydroxides are hydroxides of metals. Metal hydroxides are also known as strong bases. Many common metal hydroxides are made up from hydroxide ions and the ion of the

Table 2 from Synthesis of Me Doped Mg(OH)2 Materials for Thermochemical Heat Storage | Semantic Scholar

Complete the following equations (note that the equations are not balanced). Use the activity series - Brainly.com

⚗️Complete the following equations (note that the equations are not balanced). Use the activity - Brainly.com

Patrones de DRX de Ca(OH)2 y MWCNT G: Grafito, Ni (C): Níquel cúbico,... | Download Scientific Diagram

Alkaline thermal treatment of seaweed for high-purity hydrogen production with carbon capture and storage potential | Nature Communications

Figure 3 | Nickel and Zinc Removal from Acid Mine Drainage: Roles of Sludge Surface Area and Neutralising Agents

SOLVED: Consider these compounds: A. Fe2S3 B. Caz(PO4)2 C. Ni(CN)2 D. Ca(OH) 2 Complete the following statements by entering the letter(s) corresponding to the correct compound(s). (If more than one compound fits the

Enhanced hydrogen production via staged catalytic gasification of rice husk using Ca(OH)2 adsorbent and Ce–Ni/γAl2O3 catalyst in a fluidized bed - ScienceDirect

SOLVED: Use the activity series to determine which of these reactions will NOT happa All of these reactions will happen ZnCl2 Ba BaClz Zn Ca Ni(OH)z Ni Ca(OH)z AI(NO3l3 Pb Pb(NO3l2 None

Figure 2 from Control of uniform nanostructured alpha-Ni(OH)2 with self-assembly sodium dodecyl sulfate templates. | Semantic Scholar
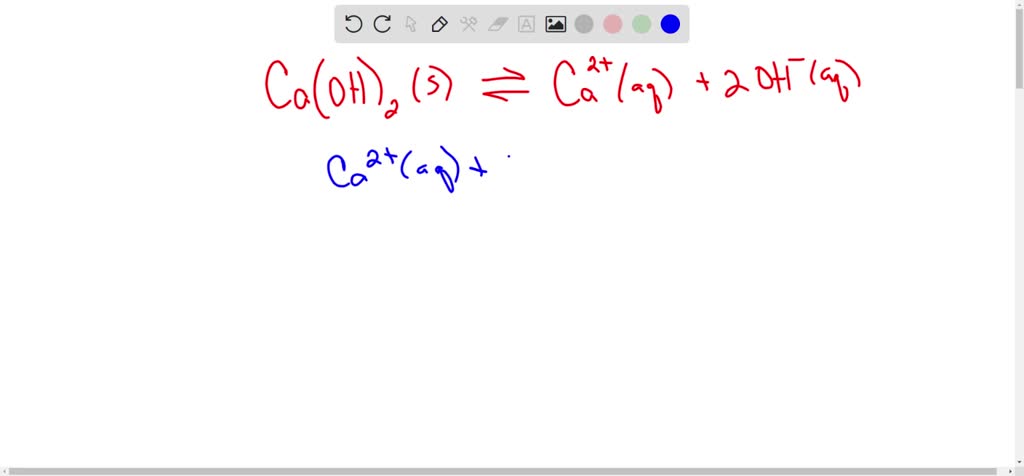
SOLVED: A saturated solution of Ni(OH)2 has the following equilibrium. Ni(OH )2 Ni2+ + 2OH- If NaOH is added to this saturated solution, the amount of Ni (OH)2 in the solution 1) increases as