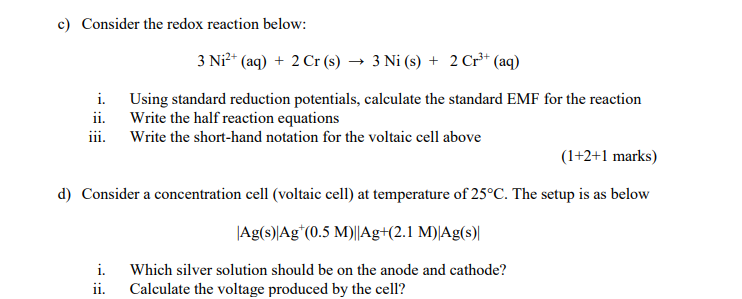
![i) Write the IUPAC name of the complex [ Cr (NH3)4Cl2 ] Cl .(ii) What type of isomerism is exhibited by the complex [ Co (en)3 ]^3 + ?( en = ethane - i) Write the IUPAC name of the complex [ Cr (NH3)4Cl2 ] Cl .(ii) What type of isomerism is exhibited by the complex [ Co (en)3 ]^3 + ?( en = ethane -](https://dwes9vv9u0550.cloudfront.net/images/3015405/99fcb6fb-74fb-4941-944b-9d7ecaa533c9.jpg)
i) Write the IUPAC name of the complex [ Cr (NH3)4Cl2 ] Cl .(ii) What type of isomerism is exhibited by the complex [ Co (en)3 ]^3 + ?( en = ethane -
Calculated Ni-Cr phase diagram as obtained in the present work. Note... | Download Scientific Diagram
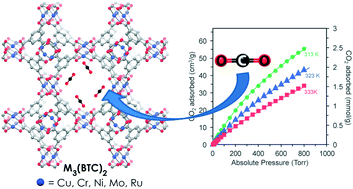
Investigation of the synthesis, activation, and isosteric heats of CO2 adsorption of the isostructural series of metal–organic frameworks M3(BTC)2 (M = Cr, Fe, Ni, Cu, Mo, Ru) - Dalton Transactions (RSC Publishing)

Description of the C11 b (oP6) phase of Pt 2 Mo-type, as occurring in... | Download Scientific Diagram

For the redox reaction `Cr_(2)O_(7)^(-2)+H^(+)+Ni rarr Cr^(3)+Ni^(2+)+H_(2)O` The correct coefficent - YouTube
in which of the following complex pairing of (n 1)d electron is not present in presence of strong field ligands 1.[Ni(NH3)6]+2 2.[Cr(CN)6] 3 4.Cr(NH3)6]3+ 4, ALL OF THESE

SOLVED: Consider the following reaction at 298K 2 Cr3+ (aq) + 3 Ni (s) 2 Cr (s) + 3 Ni2+ (aq) Which of the following statements are correct? Choose all that apply

Suppressed Growth of (Fe, Cr, Co, Ni, Cu)Sn2 Intermetallic Compound at Interface between Sn-3.0Ag-0.5Cu Solder and FeCoNiCrCu0.5 Substrate during Solid-state Aging | Scientific Reports

A novel process for preparing Fe–Cr-Ni-C alloy: synergetic reduction of stainless steel dust and laterite nickel ore | SpringerLink
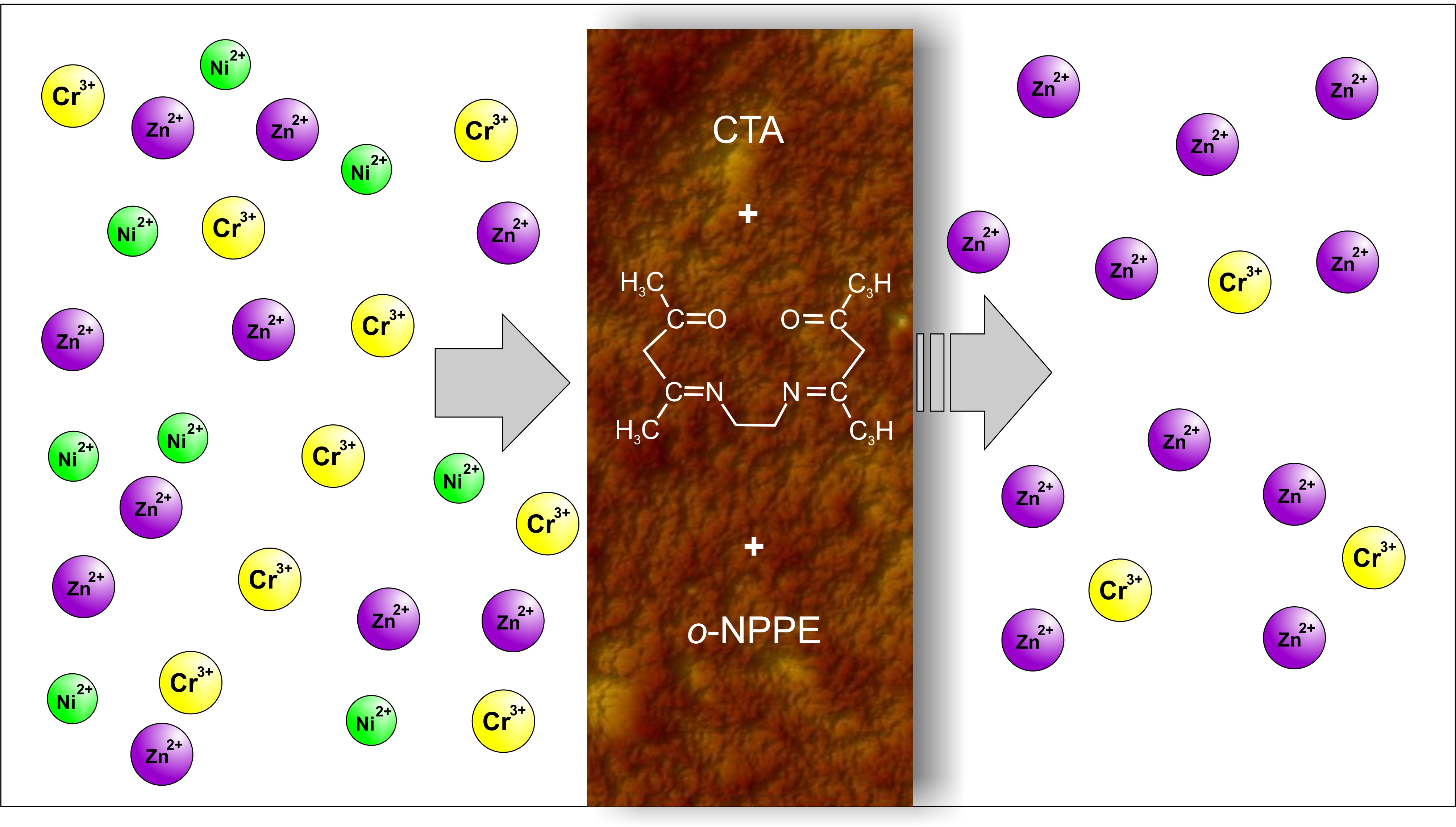
Membranes | Free Full-Text | Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier
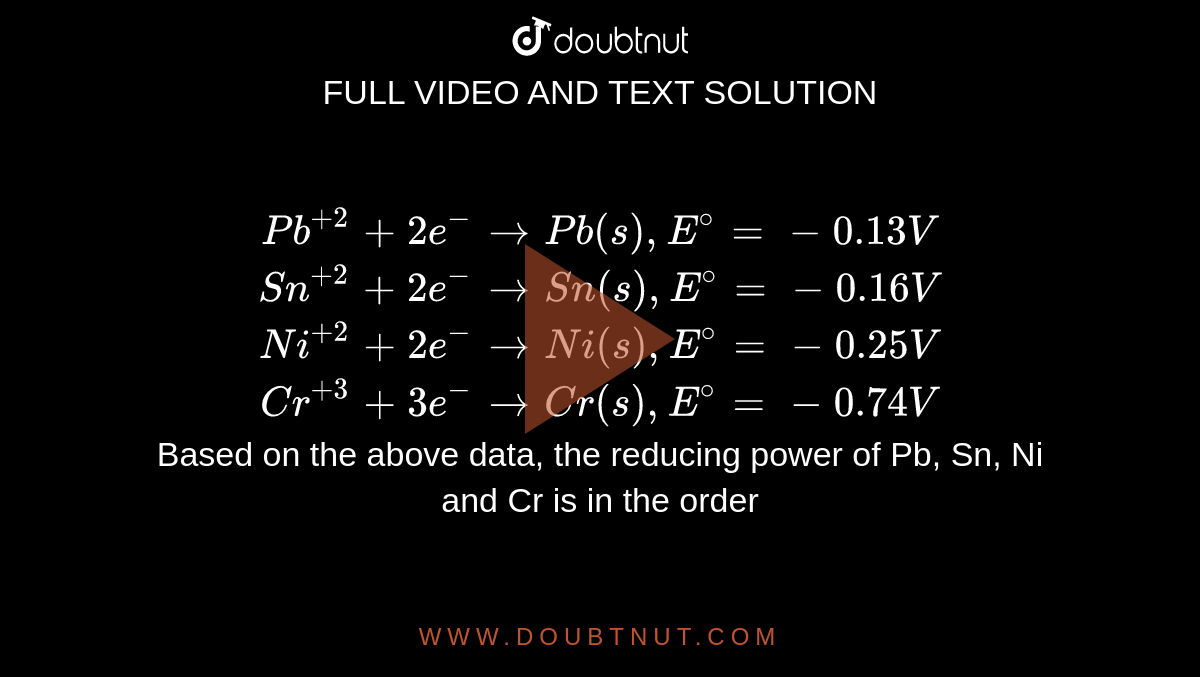
Pb^(+2) + 2e^(-) rarr Pb(s), E^(@) = -0.13V Sn^(+2) + 2e^(-) rarr Sn(s), E^(@) = - 0.16V Ni^(+2) + 2e^(-) rarr Ni(s), E^(@) = -0.25V Cr^(+3) + 3e^(-) rarr Cr(s), E^(@) = -

Coatings | Free Full-Text | Electrodeposition of Thick and Crack-Free Fe-Cr- Ni Coatings from a Cr (III) Electrolyte